by Dr John A. Wilkinson, Middlesex University, UK
Herbal medicines have great potential in the emerging nutraceutical industry in that these materials are often considered foods as well as medicines and are used in preventative and curative treatments throughout the world. Although there is a large amount of anecdotal evidence supporting the use of herbal medicines, the scientific studies to support these claims is in its infancy in many cases. One of the problems is that the scientific work that has been carried out is often published in obscure journals or by groups that do not specialise in herbal medicines but are interested in the subject peripherally. For example, the work may come from research groups that are looking for drug leads from plants rather that the use of the plant itself. Often this kind of research can highlight the fact that a particular plant may contain active constituents but it usually does not prove that the herbal medicine will be effective clinically or clarify its potential as a nutraceutical.
New plant drug development programmes are traditionally performed by either random screening or an ethnobotanical approach, a method based on the historical medicinal /food use of the plant. One reason why there has been resurgence in this area is that conservationists have argued that by finding new drug leads from the rainforest, the value of the rainforests to society is proven and that this would prevent these areas being cut down for unsustainable timber use. However Tropical forests have produced only 47 major pharmaceutical drugs of world - wide importance. It is estimated that 328 potential drugs of major importance may still be hidden somewhere. These 328 drugs would be worth $147 billion. It is thought that 125,000 flowering plant species are of pharmacological relevance in the tropical forests. It takes 50,000 to one million screening tests to discover ONE profitable drug. (Balick and Memdelsohn) Conservationists are asking: Is it worth saving the forests for this amount rather than cutting them down? However if we search for new herbal medicines rather than new active compounds for drug leads, then potentially at least 125,000 herbal medicines are to be discovered from the rainforests alone.
Even in developed countries there is huge potential for the development of nutraceuticals and pharmaceuticals from herbal materials. For example the UK herbal materia medica contains around 300 species whereas the Chinese herbal materia medica contains around 7000 species. There is therefore plenty of opportunities for future developments including specific morphological plant products, i.e. use of the fruit rather than the leaf or root, which often have different phytochemical profiles and therefore different therapeutic potential.
Consumers have shifted to the middle classes and elderly who are looking for "natural medicines" and maintenance of health.
Asia 2.3 Billion
Japan 2.1 Billion
North America 1.5 Billion
Average growth rate world-wide is around 12 - 15 % (Institute of Medical
Statistics)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Main therapeutic categories for Herbal Products
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
J. Grunwald, Herbalgram p60, 1994
Specific example: Sales of St Johns Wort, Hypericum perforatum
|
|
|
|
|
|
|
|
|
|
|
|
* Only 36 reported side effects in 5 years.
Increase in sales due to increase in research findings
![]() Used for over 2000 years by Japanese
and Chinese
Used for over 2000 years by Japanese
and Chinese
![]() Known by the Chinese as "The Medicine
of Kings"
Known by the Chinese as "The Medicine
of Kings"
![]() Known for centuries as a cardiotonic
herb
Known for centuries as a cardiotonic
herb
The plants main active phytochemicals are: polysaccharides and triterpenoids
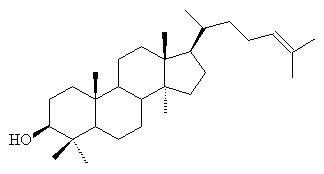 Lanosterol
Lanosterol
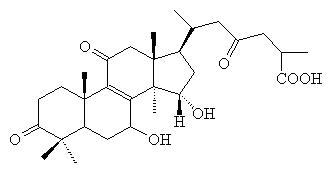 Ganodermic
acid A
Ganodermic
acid A
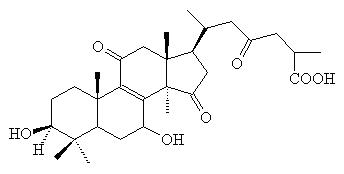 Ganodermic
acid B
Ganodermic
acid B
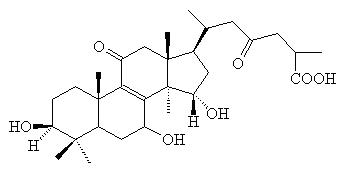 Ganodermic
acid C
Ganodermic
acid C
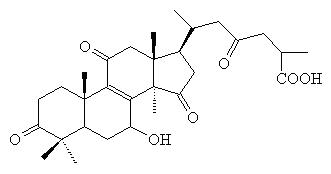 Ganodermic
acid D
Ganodermic
acid D
Researchers in China and Japan have demonstrated that the extract:
![]() Inhibits platelet aggregation
Inhibits platelet aggregation
![]() Lowers blood pressure (6 month clinical
trial : 47% of hypertensive patients lowered their blood pressure by 10
-19 mmHg, 10% by 20-29%)
Lowers blood pressure (6 month clinical
trial : 47% of hypertensive patients lowered their blood pressure by 10
-19 mmHg, 10% by 20-29%)
![]() LDL levels dropped by 68% of 90 patients
following 4 months of ingesting Reishi
LDL levels dropped by 68% of 90 patients
following 4 months of ingesting Reishi
![]() Historically the plant has been reported
to relieve pain and inflammation in the joints.
Historically the plant has been reported
to relieve pain and inflammation in the joints.
![]() The extract has been shown to have powerful
anti inflammatory effects in vitro and in vivo.
The extract has been shown to have powerful
anti inflammatory effects in vitro and in vivo.
(Journal of Chinese Medicine, 669 -674 1992).
![]() The immune stimulating properties of
the extract have been attributed to the polysaccharides.
The immune stimulating properties of
the extract have been attributed to the polysaccharides.
Ganodermic acids (oxygenated triterpenoids) present in the extract are implicated in platelet inhibition, lowering LDL and anti inflammatory processes
![]() Herb acts at the pituitary gland
Herb acts at the pituitary gland
![]() Release of follicle stimulating hormone
(FSH) is blocked while the secretion of luteinising hormone (LH) and prolactin
is stimulated
Release of follicle stimulating hormone
(FSH) is blocked while the secretion of luteinising hormone (LH) and prolactin
is stimulated
![]() Net effect: shift in the balance in
the production of oestrogen to progesterone is stimulated.
Net effect: shift in the balance in
the production of oestrogen to progesterone is stimulated.
![]() Evidence from clinical trials support
a progesterogenic effect, but many were not randomised double blind trials.
(Herbal Medicines 1994 Anderson and Phillipson)
Evidence from clinical trials support
a progesterogenic effect, but many were not randomised double blind trials.
(Herbal Medicines 1994 Anderson and Phillipson)
![]() In vitro work on the extract
has demonstrated inhibition of prolactin secretion from rat pituitary cell
cultures (Zeitschrift fur Phytotherapie 12, 77 - 82, 1991)
In vitro work on the extract
has demonstrated inhibition of prolactin secretion from rat pituitary cell
cultures (Zeitschrift fur Phytotherapie 12, 77 - 82, 1991)
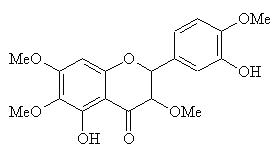 Casticin
Casticin
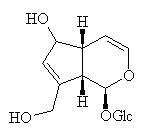 Aucubin
Aucubin
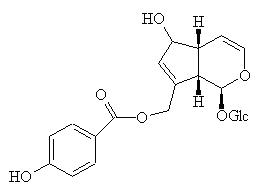 Agnoside
Agnoside
At present it is not known which constituents contribute to the activity.
Some Iridoids are known to be bitter stimulants, anxiolytics and anti inflammatory.
The above flavonoids have anti-inflammatory activity, sedative and hepatoprotective
properties. Keto steroids have been recently found.
There is also no published standard for quality control.
![]() Macroscopic and microscopic properties
Macroscopic and microscopic properties
![]() Its BOTANICAL name and family
Its BOTANICAL name and family
![]() Its geographical source
Its geographical source
![]() The method of drying and/or extraction
(including solvents used etc.)
The method of drying and/or extraction
(including solvents used etc.)
![]() Consistent analytical data (TLC, HPLC
etc.)
Consistent analytical data (TLC, HPLC
etc.)
Theoretically from a pharmacognostic point of view the above points need to be carried out in great detail, regardless of its final use, whether it is considered as a food or medicine, so that the quality of the product is known to a high degree. Once this is known then further work such as clinical trials and toxicity testing can be undertaken in the knowledge that the material being assessed is of consistent quality and therefore of consistent pharmacological activity.
NOTE: In the commercial world however the amount of data one needs will vary depending on what the regulatory requirements are.
If no claims are to be made on the product, then the herb may be considered as a food supplement rather than a medicine. Here the quality control issues again are similar but are also different! The lack of regulatory legislation puts the onus on the manufacturer/supplier to ensure that the product is not injurious to health, according to the Food Safety Act 1990. If the herbal material is well known then it maybe relatively straight forward to produce adequate safety data. If however, the company decides to introduce a herb from the Himalayas, which although has some supporting herbal folklore, is a species that is not found elsewhere in the world, then a very different scenario unfolds. The plant would be undefined and would need a full analytical and safety profile determining all the major constituents. If for example the plant contained a new class of alkaloids these would also need to be examined for safety, although it maybe possible to treat the plant extract as a whole, that is as a new chemical entity for assessment as a potential new medicine. At the end of the day, the quality control, safety and efficacy of herbal materials is very complex and each case needs to evaluated independently.
Nutraceutical companies should not be put off by this as the sale of herbal materials continues to rise and reported side effects and toxic reactions are still small compared to synthetic drugs. In some countries the regulatory bodies are becoming more open minded to allowing herbal products to be developed as nutraceuticals or even "herbaceuticals".
Recently for example, the FDA has approved a clinical trial for the treatment of Psoriasis that consists of a Chinese herbal medicine that contains 12 different plants. Similarly, the FDA has approved the human evaluation of a cancer treatment based on a crude extract from Mistletoe. This would have been unheard of 5 years ago.
In the case of Vitex previously mentioned, if no published quality
control methods are in place what is a nutraceutical company to do about
this?
The answer comes down to using established general guide lines on the
quality control of herbal materials developed by pharmacognosists and phytochemists
and apply them to the case in hand, often in consultation with an academic
laboratory. One would need to access the morphological characteristics
of the material. Is the product dried or used fresh? If dried for how long
and how. This is important as the amount of pharmacologically active compounds
in the plant can change dramatically during the drying process. This change
depends on the constituents found in the plant and also from any remaining
enzyme activity, which can break down the phytochemicals in the plant and
therefore change the phytochemical profile and subsequently the therapeutic
profile of the plant.
![]() Matricaria recutita
Matricaria recutita
Precise effects of anti inflammatory terpenoids and flavonoids. Applications
for topical/eczema
![]() Zingiber officinalis
Zingiber officinalis
Confirm efficacy in motion sickness and nausea (including post-operative)
![]() Echinacea
Echinacea
Bioactivity of constituents needs to be tied up properly. Clinical
trials needed to confirm treatment/prevention of flu/colds
![]() Ginkgo biloba
Ginkgo biloba
Establish if 24% flavonoids is the best dosage. Proper safety evaluation
needs to be carried out. Potential for memory enhancement/Altzheimers disease.
![]() Hypericum perforatum
Hypericum perforatum
Establish mode of action. Elucidate main actives and appropriate extract